THE THERMALLY ACTIVATED DELAYED FLUORESCENCE EMITTER WITH SPECIFIC CHARGE-TRANSFER EXCITED STATE AND HIGH RADIATIVE RATE CONSTANT
Сучасне матерiало- та товарознавство :: Актуальнi питання наукового та практичного матерiалознавства
Сторінка 1 з 1
 THE THERMALLY ACTIVATED DELAYED FLUORESCENCE EMITTER WITH SPECIFIC CHARGE-TRANSFER EXCITED STATE AND HIGH RADIATIVE RATE CONSTANT
THE THERMALLY ACTIVATED DELAYED FLUORESCENCE EMITTER WITH SPECIFIC CHARGE-TRANSFER EXCITED STATE AND HIGH RADIATIVE RATE CONSTANT
B. F. Minaev
V. A. Minaeva
O. O. Panchenko
B. Khmelnitsky National University, Cherkasy, Ukraine, minaeva@cdu.edu.ua
T. V. Sakhno
Poltava University of Economics and Trade
THE THERMALLY ACTIVATED DELAYED FLUORESCENCE EMITTER WITH SPECIFIC CHARGE-TRANSFER EXCITED STATE AND HIGH RADIATIVE RATE CONSTANT
The modern organic light-emitting diodes (OLEDs) are getting wide spread applications in commercially available new-generation TV sets and other flat-panel displays as well in numerous solid-state lighting lamps and toys. Since 1987 when Tang and VanSlyke had demonstrated the first fluorescent two-layered OLED the great achievements in internal quantum efficiency (IQE) increase have been obtained using phosphorescent OLED, 2–4 triplet fusion 5,6 and thermally-activated delayed fluorescence (TADF).
In present work we have considered the triphenylboron framework molecule, possessing two nitrogen sp2-hybridized atoms, which combine neighboring phenyl groups in order to construct a rigid polycyclic aromatic core [1]. This molecule called DABNA-1 was synthesized recently by prof. T. Hatakeyama at et elal. [1] in order to create charge transfer (CT) excited state without big spatial separation between donor (Nitrogen) and acceptor (Boron) atoms.
Since the nitrogen atom exhibits the opposite resonance effect of the boron atom, the para-substitution position relative to one another does enhance the resonance effects and provides significant charges separation created by excitation between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) without necessity to introduce the separated donor or acceptor groups. The most representative resonance structures of DABNA-1 molecule are shown in Figure 1. The calculated molecular orbitals of DABNA-1 are shown in Figure 2: the LUMOs are localized on the boron atom and at the ortho and para positions relative to it, whereas the HOMOs are localized on the nitrogen atoms and at the meta position relative to the boron atom.
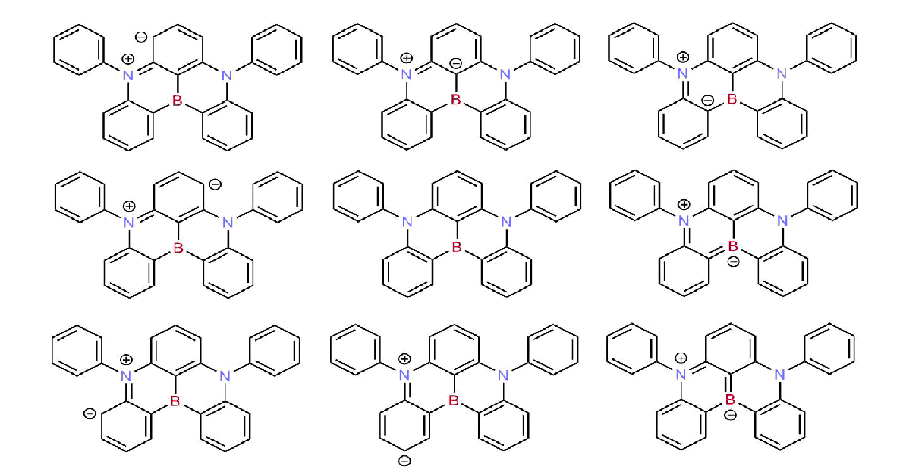
Figure 1. The most representative and important resonance structures of the DABNA-1 molecule (the right-hand analogous are omitted).
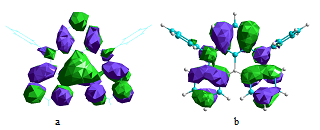
Figure 2. The HOMO orbital A′′ (a) and LUMO A′ orbital (b) of the DABNA-1 molecule.
Because of the rigid π-conjugated framework and the large oscillator strength of the S0–S1 transition (f = 0.205), OLEDs employing the DABNA-1 molecule exhibited a strong emission at 459 nm with the half-width at maximum wavelength of 28 nm only. The optimized geometry of the S0 and S1 states are quite similar; thus the Stocks shift for absorption and luminescence is rather small and the emission band is very narrow. The color of the OLED corresponds to the good CIE coordinates (the Commission Internationale de l’Elcairage) of (0.13, 0.09). The device demonstrate a high external quantum efficiency (EQE) of 13.5%.
References
1. B.F. Minaev, G.V. Baryshnikov, H. Agren. PCCP, 2014,
2. J. Xue, C. Li, L. Xin, L. Duan and J. Qiao, Chem. Sci., 2016, 7, 2888–2895.
3. Y. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238.
4. M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29,1605444.
5. I. Marghad, F. Bencheikh, C. Wang, et al. RSC Adv., 2019, 9, 4336.
6. T. Hatakeyama T., K. Shiren K. , K. Nakajima K. , S. Nomura S. , S. Nakatsuka S. , K. Kinoshita K. , J. Ni J. , Y. Ono Y. , T. Ikuta T. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect //. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect. Adv. Mater. – 2016, . – 28, . – 2777–2781.
V. A. Minaeva
O. O. Panchenko
B. Khmelnitsky National University, Cherkasy, Ukraine, minaeva@cdu.edu.ua
T. V. Sakhno
Poltava University of Economics and Trade
THE THERMALLY ACTIVATED DELAYED FLUORESCENCE EMITTER WITH SPECIFIC CHARGE-TRANSFER EXCITED STATE AND HIGH RADIATIVE RATE CONSTANT
The modern organic light-emitting diodes (OLEDs) are getting wide spread applications in commercially available new-generation TV sets and other flat-panel displays as well in numerous solid-state lighting lamps and toys. Since 1987 when Tang and VanSlyke had demonstrated the first fluorescent two-layered OLED the great achievements in internal quantum efficiency (IQE) increase have been obtained using phosphorescent OLED, 2–4 triplet fusion 5,6 and thermally-activated delayed fluorescence (TADF).
In present work we have considered the triphenylboron framework molecule, possessing two nitrogen sp2-hybridized atoms, which combine neighboring phenyl groups in order to construct a rigid polycyclic aromatic core [1]. This molecule called DABNA-1 was synthesized recently by prof. T. Hatakeyama at et elal. [1] in order to create charge transfer (CT) excited state without big spatial separation between donor (Nitrogen) and acceptor (Boron) atoms.
Since the nitrogen atom exhibits the opposite resonance effect of the boron atom, the para-substitution position relative to one another does enhance the resonance effects and provides significant charges separation created by excitation between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) without necessity to introduce the separated donor or acceptor groups. The most representative resonance structures of DABNA-1 molecule are shown in Figure 1. The calculated molecular orbitals of DABNA-1 are shown in Figure 2: the LUMOs are localized on the boron atom and at the ortho and para positions relative to it, whereas the HOMOs are localized on the nitrogen atoms and at the meta position relative to the boron atom.

Figure 1. The most representative and important resonance structures of the DABNA-1 molecule (the right-hand analogous are omitted).

Figure 2. The HOMO orbital A′′ (a) and LUMO A′ orbital (b) of the DABNA-1 molecule.
Because of the rigid π-conjugated framework and the large oscillator strength of the S0–S1 transition (f = 0.205), OLEDs employing the DABNA-1 molecule exhibited a strong emission at 459 nm with the half-width at maximum wavelength of 28 nm only. The optimized geometry of the S0 and S1 states are quite similar; thus the Stocks shift for absorption and luminescence is rather small and the emission band is very narrow. The color of the OLED corresponds to the good CIE coordinates (the Commission Internationale de l’Elcairage) of (0.13, 0.09). The device demonstrate a high external quantum efficiency (EQE) of 13.5%.
References
1. B.F. Minaev, G.V. Baryshnikov, H. Agren. PCCP, 2014,
2. J. Xue, C. Li, L. Xin, L. Duan and J. Qiao, Chem. Sci., 2016, 7, 2888–2895.
3. Y. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238.
4. M. Y. Wong and E. Zysman-Colman, Adv. Mater., 2017, 29,1605444.
5. I. Marghad, F. Bencheikh, C. Wang, et al. RSC Adv., 2019, 9, 4336.
6. T. Hatakeyama T., K. Shiren K. , K. Nakajima K. , S. Nomura S. , S. Nakatsuka S. , K. Kinoshita K. , J. Ni J. , Y. Ono Y. , T. Ikuta T. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect //. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect. Adv. Mater. – 2016, . – 28, . – 2777–2781.
Сучасне матерiало- та товарознавство :: Актуальнi питання наукового та практичного матерiалознавства
Сторінка 1 з 1
Права доступу до цього форуму
Ви не можете відповідати на теми у цьому форумі